New research shows that dietary propionate can alleviate Parkinson’s disease symptoms by enhancing gut-brain communication, offering a novel metabolic treatment strategy that bypasses the need to remove brain protein aggregates.
A study conducted by a team led by Professor Chaogu Zheng from the School of Biological Sciences at The University of Hong Kong (HKU) has unveiled that propionate, a short-chain fatty acid (SCFA), strongly suppressed neurodegeneration in animal models of Parkinson’s disease (PD) by regulating interorgan signaling between the intestine and brain.
Either inhibiting propionate breakdown or supplementing propionate through diet reversed PD-associated transcriptional aberration and enhanced energy production in the intestine, which in turn promoted neuronal health without the need of dispersing the protein aggregates.
Such metabolic rescue of neurodegeneration by increasing propionate levels provides important new insights into the treatment of neurodegenerative diseases. These research findings were recently published in a leading biology journal – Cell Reports.
Research Background
Traditional ways of treating neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) by targeting protein aggregates in the brain had very limited success, while emerging evidence suggests that metabolites derived from the gut bacteria play a critical role in modulating neurodegeneration.
PD is often characterized by the abnormal accumulation and aggregation of alpha-synuclein (alpha-syn) proteins in the dopaminergic neurons, which causes proteotoxic stress and neuronal death. Previous studies in mouse PD models found that the gut microbiota contributes to the motor deficit and neuroinflammation characteristic of alpha-syn pathology, but what microbial factors modulate host neurodegeneration is largely unclear.
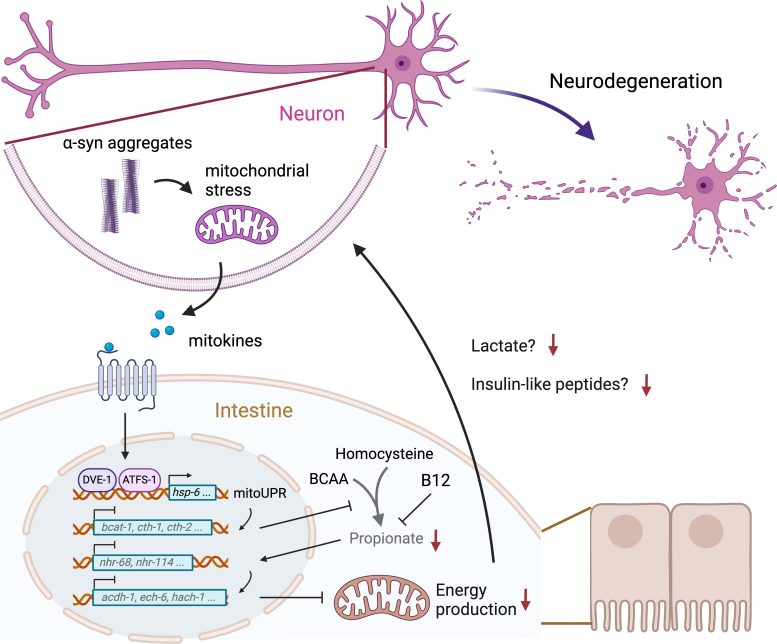
Mitochondrial stress in neurons caused by a-syn aggregation induces the secretion of signaling molecules (mitokines) that activate mitochondrial unfolded protein response (mitoUPR) and inhibit propionate production in the intestine. Reduced abundance of propionate downregulates metabolic genes, leading to deficits in energy production. Intestinal energy failure exacerbates neurodegeneration by reducing the release of lactate and insulin-like peptides. Image adapted from respective journal paper. Credit: The University of Hong Kong
One class of bacterial metabolites that have attracted a lot of attention in recent years are the SCFAs (i.e., acetic acid, propionic acid, and butyric acid) produced by anaerobic bacteria through the fermentation of dietary fiber. However, the effects of SCFAs on neurodegeneration are controversial. Some studies indicate that SCFAs exacerbate neurodegeneration and elevate inflammation, while other studies found that SCFAs protect neurons from degeneration. Moreover, the mechanisms underlying the neuronal effects of SCFAs still need to be understood.
Using a C. elegans PD model, Professor Zheng’s team previously conducted a genome-wide screen and identified 38 pro-neurodegenerative genes in E. coli. A few of these bacterial genes are essential for the biosynthesis of vitamin B12 which induces the breakdown of propionate in the host. Thus, the team hypothesized that increasing the levels of propionate may suppress neurodegeneration.
Key Findings
In this study, Professor Zheng’s team found that PD animals have lower levels of propionate than normal animals, and increasing the propionate level by either removing dietary vitamin B12 (which induces propionate breakdown) or through direct supplementation of propionate rescues alpha-syn-induced neuronal death and locomotion defects.
Surprisingly, the neuroprotective effect of propionate is mediated by interorgan signaling between neurons and the intestine. alpha-Syn aggregation in neurons triggers mitochondrial unfolded protein response (mitoUPR) in the intestine, which resulted in the reduced propionate production.
The low propionate abundance in turn caused the downregulation of numerous propionate-responsive genes involved in fatty acid and amino acid metabolisms and eventually led to defects in energy production in the intestine, which further exacerbates neurodegeneration through the gut-brain communication involving lactate and neuropeptides.
Genetically enhancing the production of propionate in the intestine or restoring the intestinal expression of key metabolic regulators downstream of propionate significantly rescued neurodegeneration, suggesting that the metabolic state of the intestine can modulate alpha-syn-induced neurodegeneration. Importantly, propionate supplementation suppresses neurodegeneration without reducing alpha-syn aggregation, demonstrating metabolic rescue of neuronal proteotoxicity downstream of protein aggregates. This new study highlights the involvement of small molecule metabolites in the gut-brain interaction in neurodegenerative diseases.
Potential Health Implications
‘This study is interesting because it connects experimental findings in animal models of PD with clinical observations. Like the PD animals, PD patients also have reduced levels of SCFAs than healthy individuals due to the reduced abundance of the commensal bacteria that produce SCFAs. Thus, the low amount of SCFAs in PD patients may indeed contribute to disease progression and severity, and supplementing propionate through the diet may help treat the disease and improve the symptom,’ said Professor Zheng, the supervisor of the research project.
Because SCFAs are produced by anaerobic fermentation of dietary fibers in the gut, Professor Zheng suggested that adding more fiber-rich food (such as seeds, nuts, fruits, and vegetables) can also increase the production of SCFAs by the gut bacteria, which may have beneficial effects on brain health.
Reference: “Metabolic rescue of α-synuclein-induced neurodegeneration through propionate supplementation and intestine-neuron signaling in C. elegans” by Chenyin Wang, Meigui Yang, Dongyao Liu and Chaogu Zheng, 26 February 2024, Cell Reports.
DOI: 10.1016/j.celrep.2024.113865





