Duke-NUS Medical School scientists have discovered the WWP2 gene’s vital role in kidney disease progression, offering a promising new target for therapies to combat chronic kidney disease and potentially save millions of lives worldwide.
The gene WWP2 regulates the energy provided to cells that cause renal failure. Drugs can be developed to inhibit this gene to slow kidney damage.
Researchers at Duke-NUS Medical School have discovered a gene, WWP2, that is key in controlling the energy flow to cells causing kidney failure. This finding presents a new avenue for developing treatments focused on reducing kidney scarring and damage.
Published in the Journal of the American Society of Nephrology, the study highlights a new approach for developing treatments and drugs to halt the progression of chronic kidney disease.
Chronic kidney disease or CKD is a global health concern contributing to high mortality rates worldwide. Singapore is particularly affected, ranking fifth in the world for new cases of kidney failure, with approximately six new patients diagnosed daily. In the advanced stage of kidney disease, kidney tissue becomes fibrotic, resulting in permanent scarring and irreversible organ damage. This condition often culminates in end-stage kidney failure, for which current treatment options are severely limited.
A New Approach to Halting Kidney Fibrosis
“Our research focused on myofibroblasts, a type of kidney cell central to the scarring of renal tissue in fibrosis. By investigating the link between the metabolic activities of these cells and the progression of the disease, we discovered that by regulating energy supply to myofibroblasts, we can control their function and potentially halt kidney fibrosis,” said Associate Professor Jacques Behmoaras from the Cardiovascular and Metabolic Disorders Programme at Duke-NUS, who is also Deputy Director of the School’s Centre for Computational Biology and co-senior author of the study.
Led by Associate Professor Enrico Petretto, director of the Centre, the research team analyzed over 130 biopsy samples from patients in China and Italy. Their findings revealed that the presence of the WWP2 gene in myofibroblasts is associated with the advancement of kidney fibrosis. The gene is crucial for regulating the mitochondria, also called the ‘powerhouses’ of the cell because they produce the energy needed for cell functions.
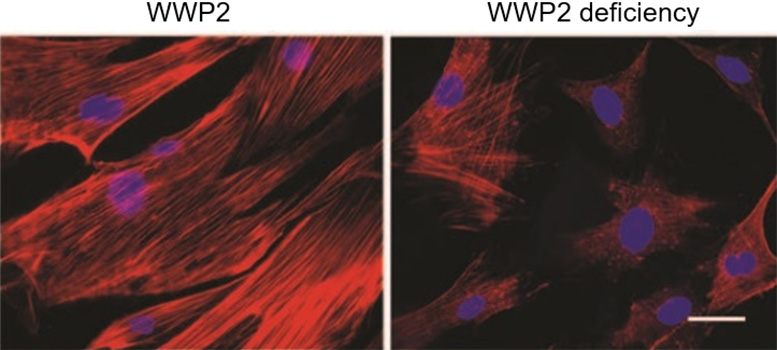
Duke-NUS scientists show that the WWP2 gene plays a critical role in advancing tissue scarring in chronic kidney disease. The panels in the image demonstrate how the presence of WWP2 results in more fibrotic tissue (left), while WWP2 deficiency results in less fibrosis (right). Credit: Duke-NUS Medical School
“In our pre-clinical models of CKD, we discovered that a higher level of WWP2 ‘re-wires’ the cell’s metabolism, contributing to the advancement of fibrosis. On the other hand, a lack of WWP2 boosts metabolism in renal cells and slows down scar formation, reducing the severity of kidney dysfunction and fibrosis,” said Dr. Chen Huimei, Principal Research Scientist with the Cardiovascular and Metabolic Disorders Programme and first author of the paper.
In earlier studies, the team had found that WWP2 controls scarring in heart disease. Targeting the gene in patients could halt the formation of excessive scar tissue and delay progression to heart failure.
Towards New Therapies for CKD
“Through our studies, we have shown that WWP2 is a new potential target for the development of drugs to halt the progression of fibrosis in several diseases. This is especially so for CKD, which can progress to renal failure and is fatal without treatment. Our findings pave the way for the design of new and promising therapies for such illnesses that would otherwise have limited treatment options,” said Assoc Prof Petretto, who is also a systems geneticist with Duke-NUS’ Cardiovascular & Metabolic Disorders Programme.
To this end, the team is in talks with venture capitalists to develop inhibitors of WWP2 to treat heart and kidney disease.
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, said: “Given the rising incidence of kidney disease in Singapore, this is a groundbreaking discovery. The study not only sheds light on the genetic mechanisms underlying kidney disease but also opens up new avenues for therapeutic intervention, offering hope for millions of CKD patients worldwide.”
Reference: “WWP2 Regulates Kidney Fibrosis and the Metabolic Reprogramming of Profibrotic Myofibroblasts” by Huimei Chen, Ran You, Jing Guo, Wei Zhou, Gabriel Chew, Nithya Devapragash, Jui Zhi Loh, Loreto Gesualdo, Yanwei Li, Yuteng Jiang, Elisabeth Li Sa Tan, Shuang Chen, Paola Pontrelli, Francesco Pesce, Jacques Behmoaras, Aihua Zhang and Enrico Petretto, 19 March 2024, Journal of the American Society of Nephrology.
DOI: 10.1681/ASN.0000000000000328





